Smart BRIDGE
to the Future Trade
Smart BRIDGE
to the Future Trade
Smart BRIDGE
to the
Future Trade
Korea International Trade Association(KITA) was established in 1946 with the objective of advancing the Korean economy through trade, and is currently the largest business organization in Korea with over 73,000 member companies.
-
0
Domestic Offices
-
0
Overseas Branches
-
0k
Member Companies
-
0
Global Network
Introduction
Korea International Trade Association(KITA) is the largest business organization in Korea with over 73,000 member companies. It was founded in 1946 to bolster the Korean economy through global trade.
KITA News
Get the latest news and updates about KITA.
Video Clip
A trustworthy partner in creating a better future.
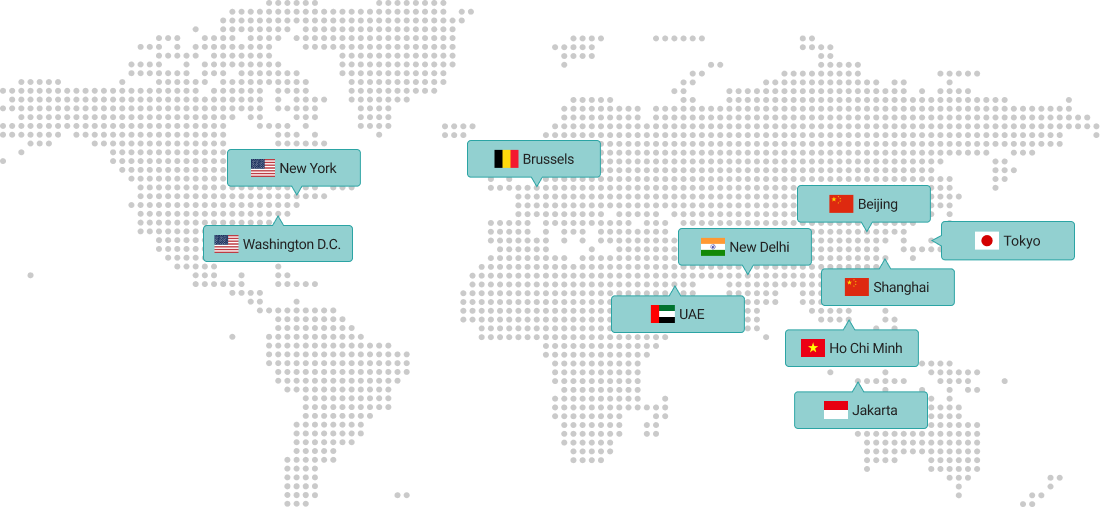
Overseas Branches
KITA is a leading business organization with a network of 13 domestic offices and 10 overseas branches in major cities.
KITA Business
KITA is the largest business organization in Korea with over 73,000 member companies.
-

Trade Policy Recommendation & Trade Consultation
-

Think Tank Specializing in Korea's Trade Strategy & Policy
-

Global Economic
Cooperation -

Education & Training :
Korea Trade Academy -

Buyer-Seller
Business Matching -
Find more about
KITA’s Core Business
-

Two way communication &
Business Support -

A bridge to foster cooperation
with global partners -

Working with 232 organizations
from 67 countries -

Ongoing management &
Regular communications -
Discover joint
business projects -
Find more about
KITA’s Global Networks
WTC Seoul
Development of an intrgrated trade, cultural & business center.
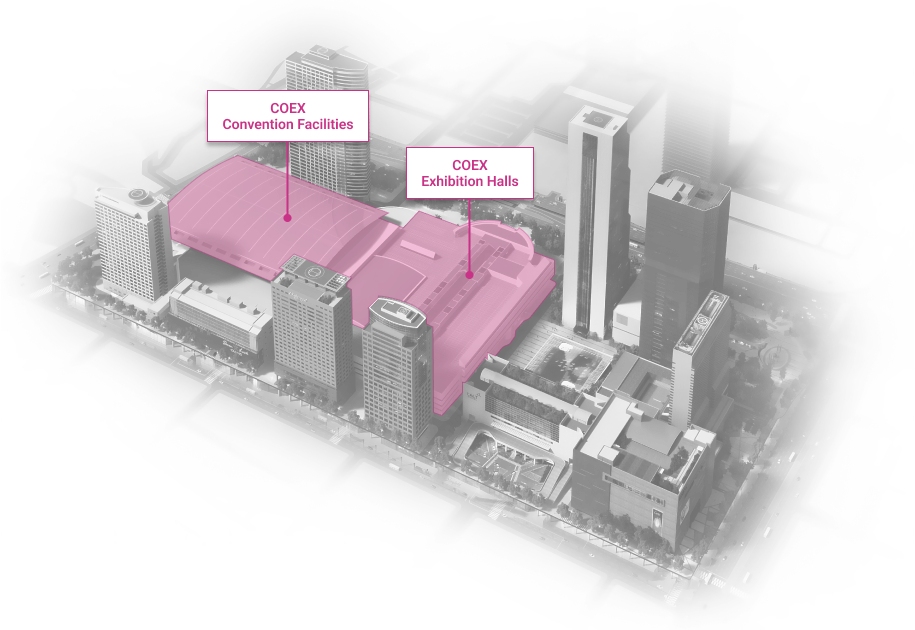
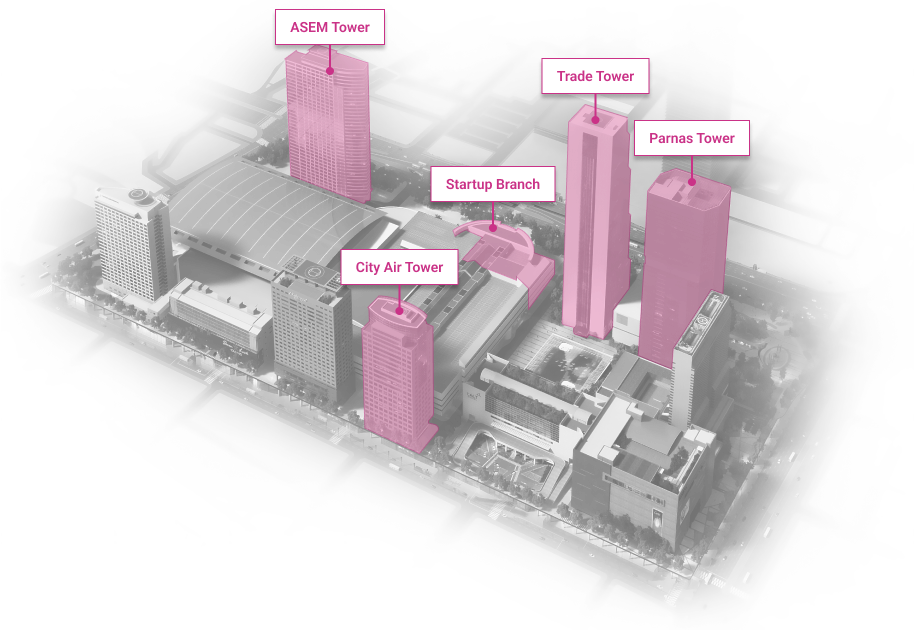
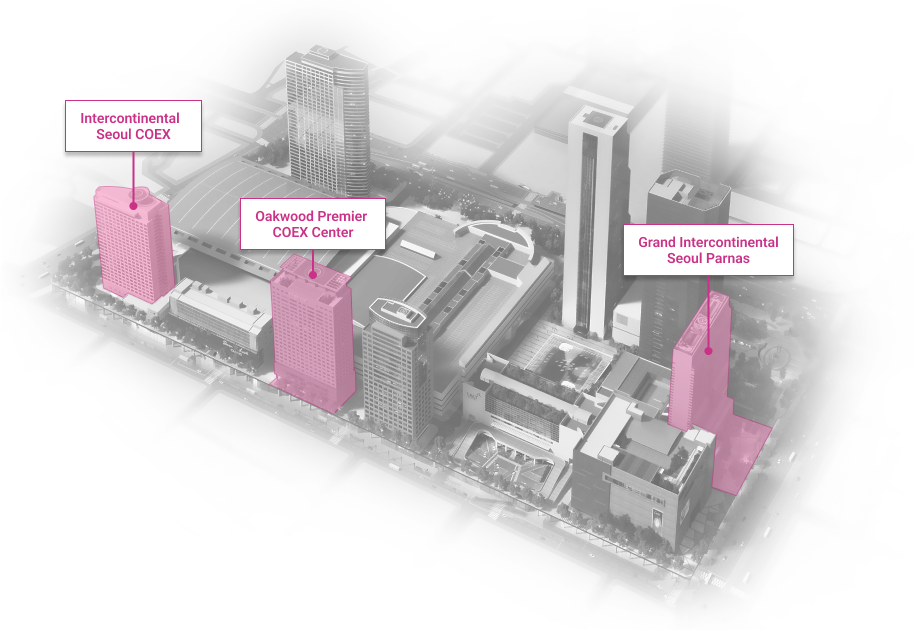

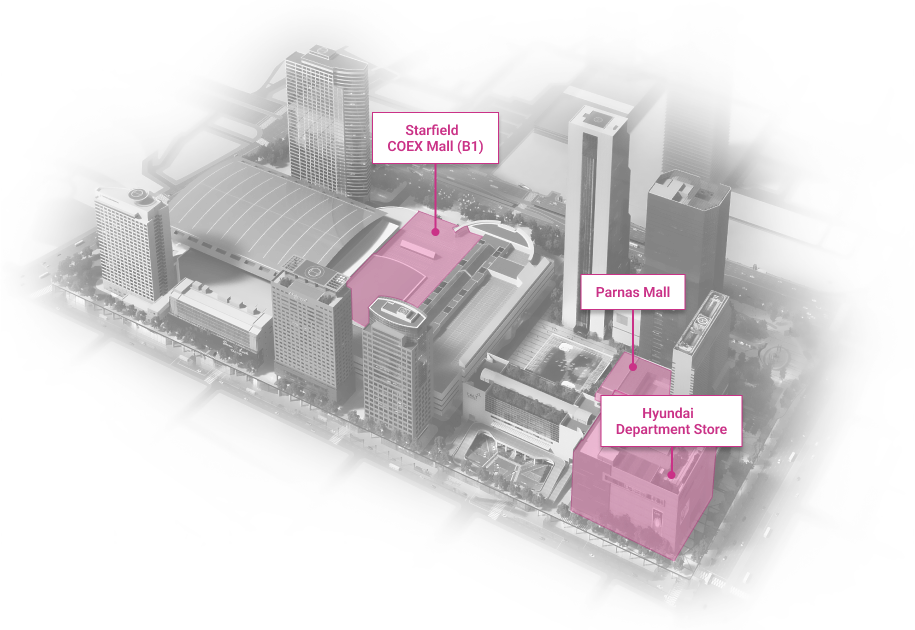
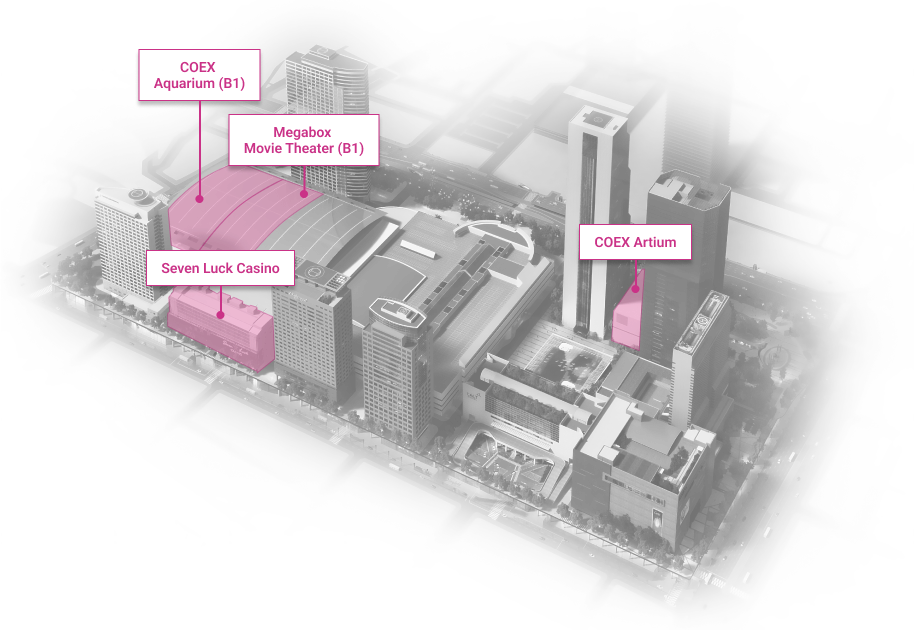
Location
Address
Trade Tower, 511 Yeongdong-daero, Gangnam-gu,
Seoul, 06164 Republic of Korea
Seoul, 06164 Republic of Korea
Telephone
(+82 2) 1566-5114